UV curable adhesives are a modern adhesive technology that offers many advantages. We at artimelt can look back on more than 20 years of experience in the development of radiation curable UV adhesives and are happy to pass on our artimelt knowledge to you for the switch to UV technology. This artimelt blog is intended to help processors of adhesives make the decision to switch to UV technology.
Change from rubber-based hot melt to UV-curable adhesives
artimelt assumes that, up to this point, you have been processing classic rubber-based hot melt. In this case the step to UV curable adhesives is a small one. You will already be familiar with hot melt, know how it is processed and have the appropriate equipment, such as a barrel melter, buffer tank and a coating plant in-house. You will also be familiar with the advantages and disadvantages of rubber-based hot melt, such as easy processing, good adhesion at low temperatures, or low weather resistance or temperature resistance. The first question to be answered now is whether you need the positive properties of UV curable adhesives for your products, such as:
- High temperature resistance up to 200°C
- High chemical resistance
- High UV resistance and therefore suitable for outdoor applications
- Approval for direct food contact
- Good punching properties
artimelt UV adhesives fulfill all these positive properties. In close cooperation, artimelt develops tailor-made UV adhesives for you, which are perfectly suited to your processing possibilities and fields of application.
Upgrade your coating systems with UV lamps
You can continue to use your existing coating system, but you will have to “upgrade” it. artimelt recommends the following: Talk to a UV equipment manufacturer and tell them what you want to do. Tell him you need UV lamps that emit in the UVC range so that artimelt adhesives can be cross-linked. Also consider the following points:
- How wide is my system?
- At what speed do I want to operate my system?
- How high is the coating weight going to be?
- How much space do I have on my existing hot melt system for UV lamp installation?
- Would I like to have the latest measurement and control technology that measures the UV output during system operation and adjusts it to the target value, or would I like to check and readjust it manually?
All these points ultimately have an influence on the entirety of the equipment and thus also on your investment costs.
Invest for the future: more UV lamps, separate barrel melting units, buffer tank and hose lines
A very important artimelt tip: Don't just have the UV system designed to meet your current needs. Think about the future and invest in more lamp units than you need today. This has the following advantages:
- You can coat a higher coating weight and network safely.
- You can increase the coating speed.
- If the existing lamp fails, you can switch on the spare lamp and continue production.
artimelt also recommends investing in separate barrel melting units, buffer tanks and hose lines to the application head. UV curable adhesives are not compatible with other hot melt systems and can lead to gel formation when mixed, resulting in a poor coating appearance.
With this additional equipment you can kill several birds with one stone:
- You can very quickly switch from classic hot melt to UV adhesives.
- You will save yourself the time-consuming cleaning of barrel melter, buffer tank and hose lines every time the adhesive is changed.
- You will avoid the risk of gel formation.
Summary
artimelt has summarized the most important points for you:
- First answer the question whether you need the properties of the UV adhesives.
- You will need lamps that emit in the UVC range.
- Think about the future and invest in more lamp units than you need today.
- Invest in additional peripheral equipment such as a barrel melter, buffer tank and hose lines.
artimelt has comprehensive know-how regarding UV adhesives and the best contacts to equipment manufacturers. Let your artimelt Key Account Manager advise you.
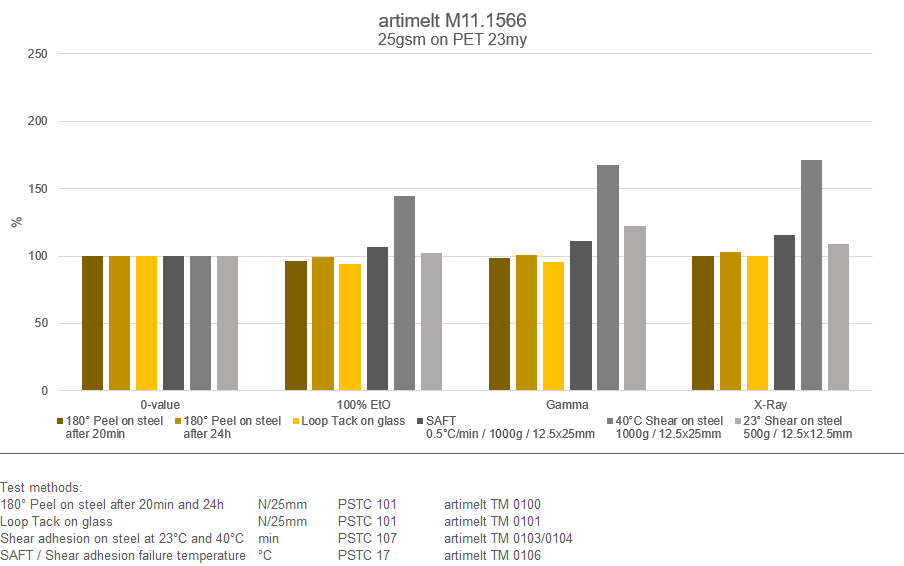 Extract from the results for the artimelt M11.1566 adhesive
Extract from the results for the artimelt M11.1566 adhesive